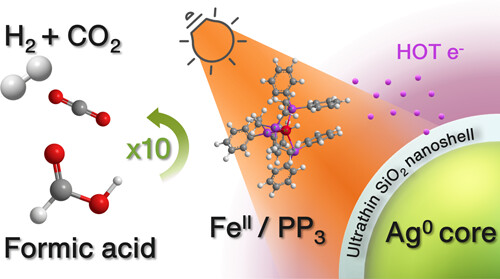
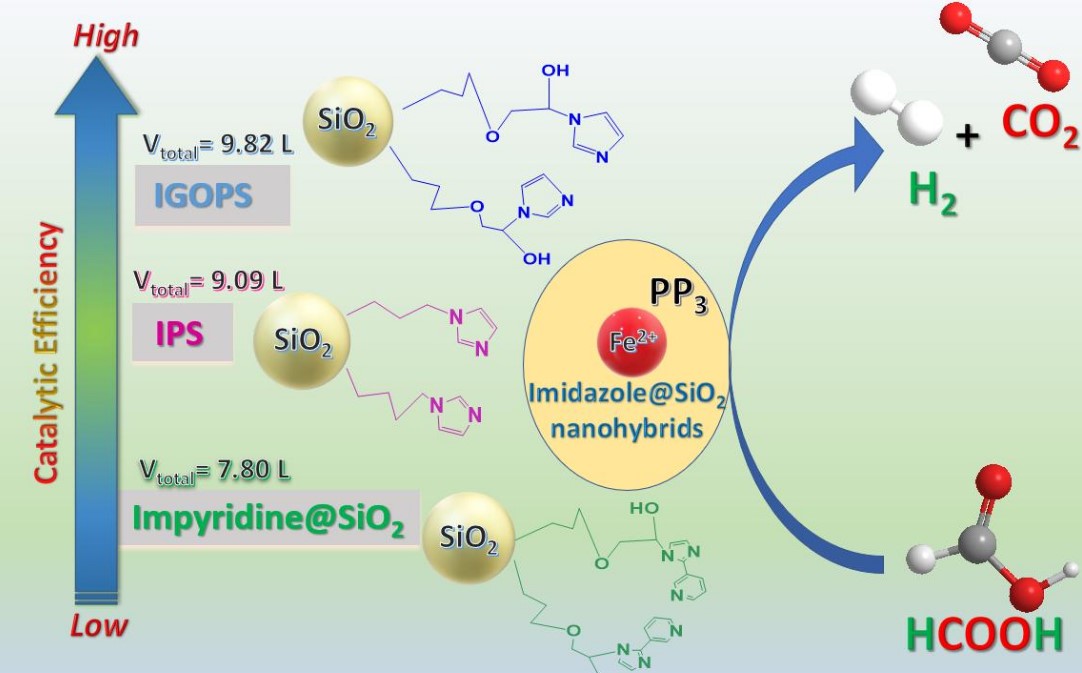
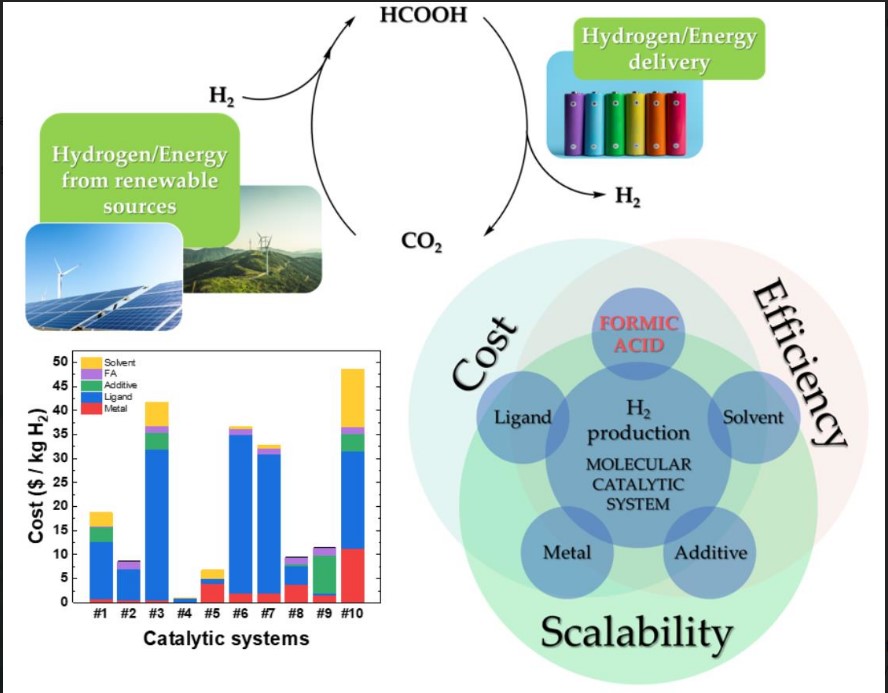
Προσκαλούνται οι ενδιαφερόμενοι να υποβάλουν εκδήλωση ενδιαφέροντος για μία θέση συνεργάτη/συνεργάτιδας, ο/η οποίος-α θα διεξάγει έρευνα με στόχο την εκπόνηση Διδακτορικής Διατριβής με θέμα που αφορά στην Καταλυτική Παραγωγή Η2 από μικρά οργανικά μόρια.
Περιγραφή ερευνητικής απασχόλησης:
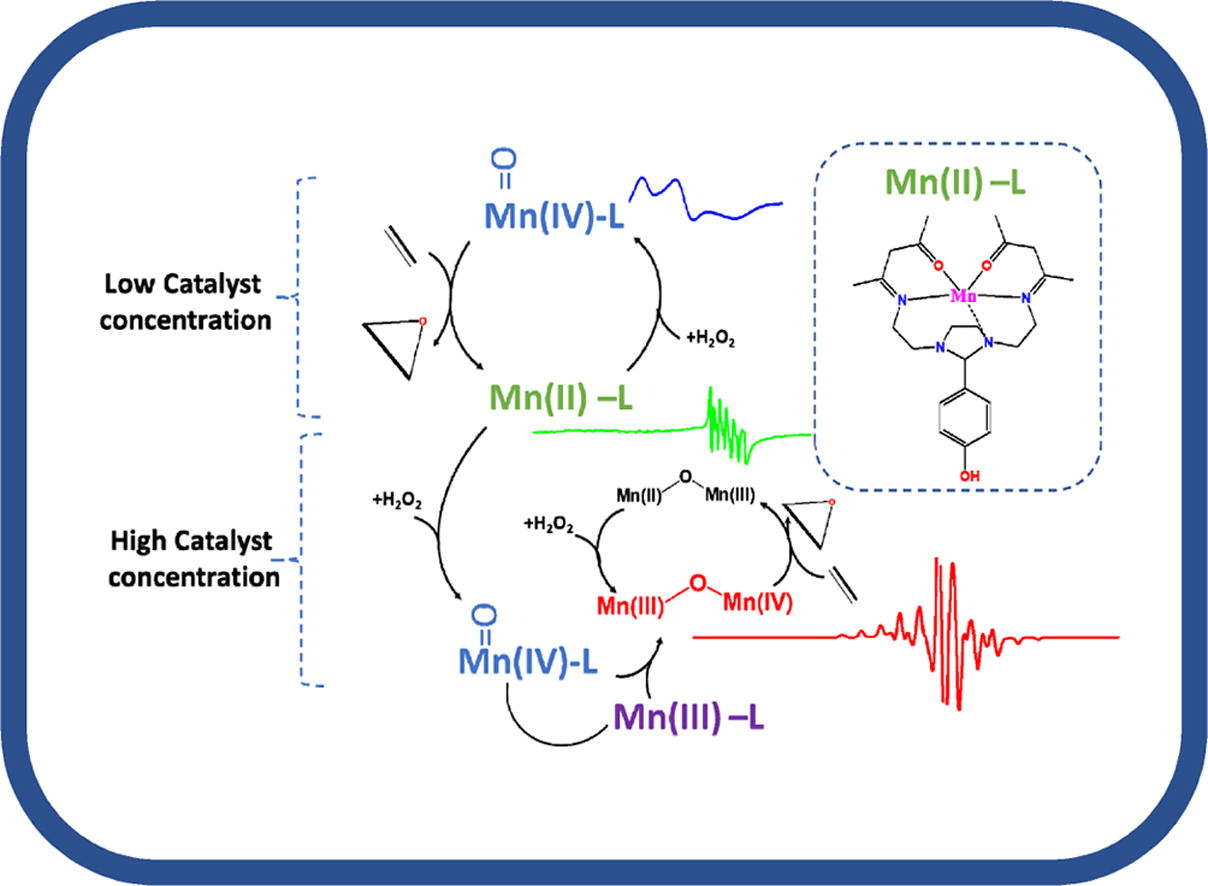
Προηγμένα υλικά για παραγωγή Υδρογόνου
Προηγμένα υλικά για α) δέσμευση και μετατροπή CO2 σε χημικά προστιθέμενης αξίας, και β) εξοικονόμηση ενέργειας σε κτίρια (ΠΟΛ.ΚΡ.)
Ο/Η υποψήφιος-α διδάκτορας που θα επιλεγεί θα εργαστεί στο Τμήμα Χημείας του Πανεπιστημίου Ιωαννίνων, με επιβλέπουσα την Καθηγήτρια Μαρία Λουλούδη (https://chem.uoi.gr/meli-dep/louloudi-maria/). Η ολοκλήρωση της ερευνητικής του εργασίας αναμένεται να οδηγήσει σε επιστημονικές δημοσιεύσεις σε διεθνή περιοδικά με υψηλούς δείκτες απήχησης.
Ο/H υποψήφιος-α διδάκτορας που θα επιλεγεί μπορεί να ξεκινήσει την ερευνητική του εργασία μόλις ολοκληρωθεί η εγγραφή του για απόκτηση Διδακτορικής Διατριβής (προβλεπόμενη ημερομηνία Φεβρουάριος 2024).
Απαραίτητα Προσόντα
Οι υποψήφιοι πρέπει να διαθέτουν Πτυχίο Χημείας ή Χημικού Μηχανικού ή Επιστήμης Υλικών ή Φυσικής και Μεταπτυχιακό Δίπλωμα Ειδίκευσης στη Χημεία ή Χημεία και Τεχνολογία Υλικών. Ερευνητική εμπειρία στην Κατάλυση και τον Χαρακτηρισμό Υλικών θα εκτιμηθούν σε μεγάλο βαθμό.
Η άριστη γνώση της Αγγλικής Γλώσσας (γραπτά και προφορικά – κατά προτίμηση επιπέδου Proficiency) είναι απαραίτητη.
Οι ενδιαφερόμενοι καλούνται να επικοινωνήσουν με την Καθηγήτρια Μαρία Λουλούδη: mlouloud@uoi.gr / 2651008418.